Results of an Oncology Clinical Trial Nurse Role Delineation Study
Purpose/Objectives: To evaluate the relevance of a five-dimensional model of clinical trial nursing practice in an oncology clinical trial nurse population.
Design: Web-based cross-sectional survey.
Setting: Online via Qualtrics.
Sample: 167 oncology nurses throughout the United States, including 41 study coordinators, 35 direct care providers, and 91 dual-role nurses who provide direct patient care and trial coordination.
Methods: Principal components analysis was used to determine the dimensions of oncology clinical trial nursing practice.
Main Research Variables: Self-reported frequency of 59 activities.
Findings: The results did not support the original five-dimensional model of nursing care but revealed a more multidimensional model.
Conclusions: An analysis of frequency data revealed an eight-dimensional model of oncology research nursing, including care, manage study, expert, lead, prepare, data, advance science, and ethics.
Implications for Nursing: This evidence-based model expands understanding of the multidimensional roles of oncology nurses caring for patients with cancer enrolled in clinical trials.
Jump to a section
The specialty practice of clinical trial nursing was recently recognized by the American Nurses Association (International Association of Clinical Research Nurses [IACRN], 2016). With this recognition came a five-year acknowledgement of clinical trial nurses’ (CTNs’) scope and standards of practice (American Nurses Association & IACRN, 2016). Nomenclature for clinical trial nursing is confusing, and precisely what CTNs do on a day-to-day basis is unclear based on the role title alone. Two nursing roles have been described in the literature—the CTN and the research nurse coordinator. When compared, CTNs were found to perform higher levels of clinical practice than research nurse coordinators (Bevans et al., 2011). The CTN’s role is the provision of direct patient care to study participants, and the research nurse coordinator’s nursing activities were more frequently related to a specific study or principal investigator (Bevans et al., 2011). Although the American Nurses Association stated that CTNs make important contributions to the research process and have specialized training in nursing care—research regulations, scientific processes, participation protection, data collection, analysis, and interpretation (IACRN, 2016)—many questions about their roles and responsibilities remain. This recognition reveals how nurses contribute to the clinical trial enterprise; however, these statements and descriptions of the CTN role may not fully represent the oncology clinical trial nursing practice. It is unclear if the designation of clinical trial nursing as a specialty practice by the American Nurses Association overlaps, and to what degree, with the roles and responsibilities of research nurse coordinators.
Patients enrolled in an oncology clinical trials present with complex emotional, medical, and educational needs, with care driven by the requirements of the trial and collection of research data (Hastings et al., 2012). As a result, the oncology CTN needs to be prepared to deliver care; however, the roles and contributions of nurses to the care of patients with cancer participating in clinical trials are not clearly described in the literature. Their job descriptions, scope of practice, and job titles also vary (Castro et al., 2011; Hastings et al., 2012; Jones & Wilson, 2013). In some clinical trial settings, the CTN is narrowly focused on coordination aspects of clinical trials, particularly on the collection of research data and the fidelity of the study; in other clinical trial settings, the CTN is primarily focused on direct patient care, administration of study drugs, and meeting the emotional needs of the patient. Still in other clinics or hospitals, the CTN role may be broader, consisting of a dual role of direct patient care and clinical trial coordination (Bevans et al., 2011; Carlson, Reilly, & Hitchens, 2005; Castro et al., 2011; Hastings et al., 2012; Nagel, Gender, & Bonner, 2010; Scott, White, Johnson, & Roydhouse, 2011; Spilsbury et al., 2008). Clinical trial participants are cared for in a variety of settings, including inpatient units, outpatient infusion centers, ambulatory care clinics, private oncologist offices, and radiation therapy facilities (Rieger & Yarbro, 2003). The nursing care provided by CTNs must meet a myriad of emotional, medical, and educational needs of patients while adhering to strict trial and research guidelines and protocols (Hastings et al., 2012). Given the experimental and medically intricate nature of oncology clinical trials, patients and their families require a high level of care and expect competent clinicians who are well versed in the care of research participants. To define the role of the oncology CTN, an agreed-upon taxonomy or classification system (Castro et al., 2011) should be developed.
One of the current authors observed that it is not uncommon for principal investigators or department administrators to ask CTN managers, “Can the clinical trial nurse perform informed consent?” or say, “I didn’t know I could ask the nurse to create a study budget.” This role confusion leaves nurses uncertain about their scope of practice and preparation (Jones & Wilson, 2013). For example, unlicensed personnel may perform activities that should be performed by nurses (Jones & Wilson, 2013), but the Code of Federal Regulations is vague on the delegation of authority by principal investigators in the conduct of clinical trials (U.S. Food and Drug Administration Responsibilities of Sponsors and Investigators Rule, 2016). The principal investigator, who is ultimately responsible for the fidelity of the clinical trial, may not know what and to whom they can delegate. Therefore, clarity is needed on the role of oncology CTNs to advance practice (Bevans et al., 2011; Ehrenberger & Lillington, 2004; Nagel et al., 2010; Spilsbury et al., 2008).
Two instruments have been developed to delineate the role of the CTN—the Clinical Trial Nurse Questionnaire created by Oncology Nursing Society’s (ONS’s) Research Nurse Special Interest Group (Ehrenberger & Lillington, 2004) and the Clinical Research Nurse Role Delineation Measure (Bevans et al., 2011; Castro et al., 2011; Ehrenberger & Lillington, 2004). Although the Clinical Trial Nurse Questionnaire was developed by oncology CTNs and was deemed valid (content validity = 0.95) and reliable (alpha = 0.92, test–retest reliability = 0.88), the theory with which this instrument was developed (Nurse Role Effectiveness Model) is incongruent with its operational measures. Additional limitations include the length of the questionnaire (12 sections and 154 items), the results’ lack of reference to the Nurse Role Effectiveness Model, and its need for updating (published in 2004). Therefore, the Clinical Trial Nurse Questionnaire was not used for this study.
The Clinical Research Nurse Role Delineation Measure survey (Bevans et al., 2011) was the result of a five-dimensional model theorized using expert consensus to categorize clinical trial nursing activities (Castro et al., 2011). The dimensions conceptualized by nursing experts at the National Institutes of Health are care coordination and continuity, clinical practice, contributing to the science, human subject protection, and study management. Within each dimension, specific nursing activities were proposed. The Clinical Research Nurse Role Delineation Measure was created based upon the activities proposed by this group of nursing experts. In a test of this model, the National Institutes of Health researchers sampled 412 oncology, behavioral/mental health, medical/surgical, critical care, and perioperative/perianethesia nurses with clinical trial roles (Bevans et al., 2011). This study did not report the variance of each dimension; therefore, it is unclear to what extent this model explains the role of CTNs in this sample. Two distinct roles of the CTN and clinical trial coordinator were described, and the authors recommended additional study using a large, national sample of nurses (Castro et al., 2011; Chang, Gardner, Duffield, & Ramis, 2012).
Methods
To build upon the literature and better understand the dimensions of practice of the oncology nurse in research, the current study was undertaken. The objective of this study was to evaluate the relevance of the five-dimensional model of clinical trial nursing practice in an oncology clinical trial nursing population. Survey data were collected from a sample of oncology nurses who care for clinical trial participants from all regions of the United States to test the use of the five-dimensional model. Participants were asked to self-report if they met eligibility criteria by answering two questions. Participants were eligible for the study if they were currently practicing in the United States and working in oncology clinical trials providing direct patient care, trial coordination, or a combination of both. Participation was voluntary and anonymous. Informed consent was presumed based on commencement of the survey. Institutional review board approval was obtained from the University of Texas at Tyler.
Sample
A convenience sample of 167 oncology nurses employed in the United States working in clinical research (direct patient care, coordination of a clinical trial, or both) were recruited by using targeted advertisements on social media (e.g., Facebook, Twitter, LinkedIn) and emailing potential participants (email addresses were obtained from professional databases, including the ONS Clinical Trial Nurse Special Interest Group, the IACRN, and the researcher’s professional oncology nursing network). Email invitations and direct solicitations through social media provided an overview and study purpose. Potential participants from both pools were directed to the web-based Qualtrics survey tool. The survey took an average of 15 minutes to complete and was open from December 2015 to February 2016.
Instrument
The Clinical Research Nurse Role Delineation Measure was used with permission (Bevans et al., 2011). A 12-question survey was used to characterize participant demographics. No personal identifiable information was collected. Participants were given a list of 59 activities and were asked to answer how frequently they engaged in each of them on a scale of 1 (not part of my practice) to 6 (multiple times a day) as it related to their current role. This scale performed well in a previous study with a Cronbach alpha reliability for the frequency items of 0.95 (Bevans et al., 2011).
Analysis
Data were downloaded and imported into SPSS®, version 21. Descriptive statistics were calculated depending on the distribution of each variable. For continuous variables, histograms, means, and standard deviations were generated to assess normality. For ordinal variables, medians and ranges were calculated. Tabulations were used for categoric variables.
Principal Components
Principal components analysis (PCA) was used to determine the dimensions of practice for oncology CTNs. PCA is a data-reduction technique used to examine the interrelated nature of variables, extract them, and reduce them for further assessment (Abdi & Williams, 2010). The goal of PCA is not only to determine the number of underlying dimensions of a construct (in this case, clinical trial nursing) but to determine how well the dimensions explain the construct. In this way, the PCA technique generates new variables, called “principal components,” which represent combinations of the original variables (Abdi & Williams, 2010). The first principal component would explain the largest proportion of total variance in the original variables explained by the component; the second extracted component would explain less variance and is statistically independent (i.e., orthogonal) from the first component (Abdi & Williams, 2010).
The initial steps in planning a PCA is to select a model for testing. The five-dimensional domain of practice for CTNs was selected for testing in the current study. PCA was selected with varimax rotation to determine the dimensions of nursing practice. Scree plots and corresponding eigenvalues were used to determine the number of components in the data, and varimax rotation was used to examine the stability of the results of frequency scores from the survey. After the number of components was identified, inter-item matrices were used to classify each item into a principal component based upon the following guidelines: a loading greater than 0.6 on a single component and a loading less than 0.3 on each other component. Cronbach alpha was calculated to evaluate internal consistency of each newly generated scale.
Results
Demographics
Most nurses participating in the study practiced for 20 or more years, were aged 50–59 years, worked full-time, and had been in their research role for five years or more. Most study participants had a Bachelor’s degree and cared for adults (see Table 1). The sample included participants from all over the United States. The three groups of nurses self-identified as primarily involved in direct patient care, primarily involved as a trial coordinator, or involved in patient care and clinical trial coordination. The largest group (n = 91) had dual roles of direct patient care and trial coordination, followed by coordinators (n = 41) and direct care clinicians (n = 35).
[[{"type":"media","view_mode":"media_original","fid":"39316","attributes":{"alt":"","class":"media-image","height":"859","typeof":"foaf:Image","width":"371"}}]]
Principal Components Analysis
PCA was used to replicate the five-dimensional clinical research nursing domain of practice for the oncology nurse who cares for patients enrolled in clinical trials. The frequency scales met the criteria set by Field (2009) for the Kaiser-Meyer-Olkin (Kaiser, 1974) values of 0.92 and a significant Bartlett’s test of sphericity indicating that the data were amenable to PCA with varimax rotation. Eight unique components containing 51 variables were extracted (see Figure 1). These components explained 64.12% of the total variance in the original variables. The eight factors were classified and labeled as care (20 items, alpha = 0.957), manage study (5 items, alpha = 0.914), expert (8 items, alpha = 0.855), lead (6 items, alpha = 0.81), prepare (4 items, alpha = 0.771), data (3 items, alpha =0.755), advance science (3 items, alpha = 0.765), and ethics (2 items, alpha = 0.784). The dimension of care explained most of the role (37.6%) compared to the other factors. 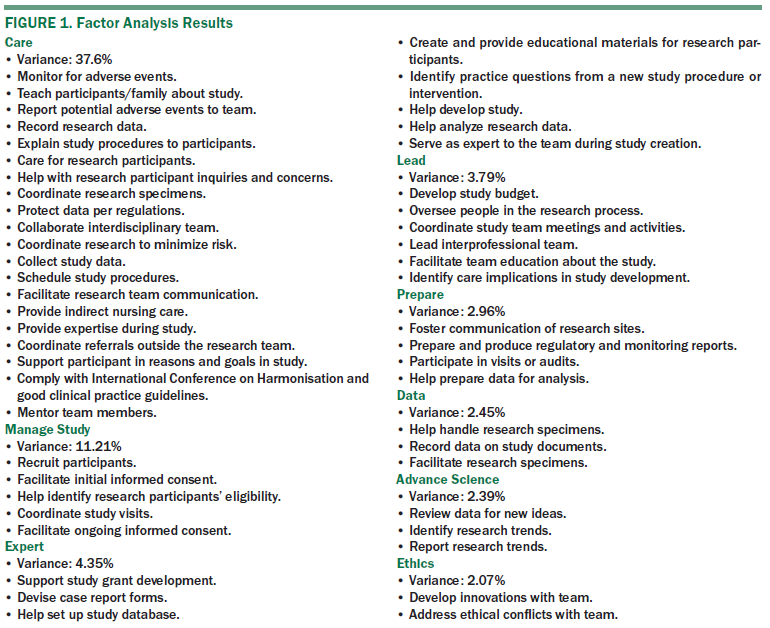
Discussion
Domains of Practice
The primary purpose of this study was to replicate and expand characterizations of oncology clinical trial nursing responsibilities from previous studies. Results of the current study suggest that nurses’ practice responsibilities are more multidimensional than previously anticipated (Bevans et al., 2011). Specifically, the current results support an eight-dimensional model of oncology clinical trial nursing practice versus the five-dimensional model of care theorized previously. This eight-dimensional model of care is based on the results of the factor analysis of the frequency data from the survey, whereas the five-dimensional model was based on national expert opinion (Castro et al., 2011).
Activities in this eight-dimensional model of care also were grouped quite differently than the activities in the five-dimensional model. The same activities were used in both studies to operationalize the oncology nurse’s role, which may be implemented differently. The eight-dimensional model suggests that oncology CTNs spend most of their time caring for patients (20 activities and 37.6% of the variance), contrary to popular perceptions of them providing more administrative tasks. In the five-dimensional model, care coordination included 14 items and explained variance was not provided. The care dimension included activities like monitoring patient for adverse events, teaching participants about the study, reporting potential patient adverse events, recording patient research data, explaining study procedures to patients, and directing patient care to research participants.
In addition, evidence from the current study suggests that nurses’ scope of practice extends beyond patient care to activities (e.g., the lead dimension, which includes the development of study budgets, oversight of people in the research process, and coordination of team meetings). The expert dimension includes activities that call upon the nurse’s expertise, such as support of study grants, creation of case report forms, assistance in setting up study databases, creation of educational materials for research participants, and identification of practice questions related to a novel therapy or intervention under study.
The eight-dimensional model has additional dimensions—expert, lead, prepare, and data—that were not included in the originally proposed five-dimensional model, and each dimension has multiple activities. Many of the activities that created the care dimension were in the clinical practice dimension from the originally proposed five-dimensional model. It is difficult to draw conclusions as to why the activities grouped differently because the statistical technique for grouping activites varied between studies. In addition, the sample population differed from that in previous studies. The labels for this analysis were created after examination of the activities within each dimension, then a broad label was chosen to represent those activities.
The multidimensional domains of practice presented in this study underscore the complex nature of the oncology CTN role. The diversity of roles and responsibilities assigned to oncology nurses are complex, leaving these nurses at risk for role confusion. Oncology nurses involved in the care of patients receiving experimental therapies are expected to be autonomous in roles driven by the clinical trial protocol. The potential for role conflict should be examined in additional studies to further define and clarify nurses’ scope of practice.
Strengths and Limitations
Results from this study should be considered in light of the limitations, including a small sample size, the use of convenience sampling rather than a probability sample, the potential ineligible participants enrolled because of self-selection, and the anonymous nature of the survey. Although the current sample was smaller than that in previous studies (Bevans et al., 2011), it was demographically more diverse, consisting of nurses employed in oncology clinics across the United States, which could have affected the findings. Previous study participants were employed at a single institution and worked in many clinical specialties (not only cancer trials). The larger sample size in the present study permitted the use of the factor analytic procedure, which cannot generate stable results with small samples. These data were factorizable, and the robust statistical technique for grouping the activities into dimensions of practice helped control for the limited sample size.
Recruitment for this study was conducted using social media and email to educate nurses on eligibility and decrease the chances of enrollment of ineligible participants. It is difficult to determine if the study sample is demographically representative of oncology CTNs in the United States because no previous studies or reports of a national sample are available. The current study sample was demographically similar to samples in previous studies (Bevans et al., 2011) in age distribution, gender, years as a nurse, years practicing in their current role, and educational preparation; however, the current sample included slightly more postgraduate nurses.
Social desirability in response to questions regarding caring or ethics may have affected participant responses, but this threat was controlled by use of an anonymous web-based survey. Although self-reported frequency measures were not ideal because of the risk for recall bias, the bias would likely be limited because nurses were currently working in a clinical research setting and executed these responsibilities daily.
The strengths of this study include the robust statistical technique used to determine the theoretical dimensions of practice. In addition, the oncology nurses were recruited from various research settings across the United States. In this study, nurses with dual roles, serving as study coordinators and providing direct care, represented most of the sample. Historically, this role was not well understood. Past role delineation studies of CTNs dichotomized this population to either direct patient care providers or study coordinators, leaving out this important dually trained group of nurses (Bevans et al., 2011). The inclusion of community oncology research practice settings, large comprehensive cancer centers, and academic institutions likely contributed to a more diverse sample.
Implications for Research
Additional studies to contextualize oncology CTN practice are needed. Quantitative results from this study provide evidence of the diverse roles oncology CTNs are charged with. These findings contribute to the literature by providing an evidence-based description of the role of the oncology nurse in the care of patients enrolled in clinical trials. Because of the complex nature of the role and the rapidly evolving landscape, oncology clinical trial nursing should be examined in future studies. Qualitative studies may provide evidence that cannot be captured through anonymous survey techniques, such as role confusion or feelings related to autonomy to perform the role. Care of patients enrolled in oncology clinical trials includes intangible emotional preparation and skills that may be better captured and reported in qualitative studies. In addition, studies that explore nurses’ perceptions of autonomy in clinical trials may increase the understanding of nurses perceived control in their practice and enhance support for them.
Future studies should replicate these findings using larger, probabilistic samples of nurses from various oncology practice settings and locations to ensure that the full scope of clinical trial nursing practice is documented. Direct care providers, clinical trial coordinators, and those with dual roles should be included in the studies. In addition, an understanding of the various roles nurses have under different forms of government and healthcare systems is needed to expand this knowledge to nurses working overseas. Oncology clinical trials are becoming more complex, and with increased awareness and government support for cancer clinical trials because of the Cancer Moonshot initiative, nurses must be well prepared.
Implications for Nursing
This study clarifies the dynamic roles of oncology CTNs. To advance practice, the nursing community must continue to use validated statistical techniques to inform clinical practice. This model and these data are foundational to the professional advancement of nurses. With additional research to support its validity and use in other samples, the eight-dimensional model could be used as the basis for workforce development, competency development, and scopes and standards of practice. The results of this study can be used by oncology nurse employers to better understand the service profile; build high-quality, more accurate job descriptions; and assist with performance evaluations and salary justification. In addition, competencies should be built upon domains or broad categories of knowledge, and this eight-dimensional model of oncology clinical trial nursing can be used as a foundation to grow competencies. 
Conclusion
Nurses are well positioned to empirically define their role and contributions to the care of patients with cancer enrolled in clinical trials. All oncology nurses who care for patients enrolled in clinical trials have multidimensional roles ranging from patient care and study management to budget development and data management. Nurses should have control over nursing practice, and an evidence base supporting clear dimensions of practice is an important step. Future studies should replicate these findings in other settings and samples, and contextualize the dimensions of practice identified in this study using qualitative research.
The authors gratefully acknowledge Sally Northam, PhD, for serving as dissertation chair and mentor and Clare Hastings, PhD, for serving as dissertation committee member and mentor.
About the Author(s)
Purdom is senior director of Clinical Development/Medical Affairs in oncology at TG Therapeutics based in Houston, TX; and Petersen is a professor and director of the DNP program in the School of Nursing and Haas is an associate dean of the College of Nursing and Health Sciences and executive director of the School of Nursing, both at the University of Texas at Tyler. Purdom was supported by a grant from Houston Chapter of the Oncology Nursing Society. Purdom and Haas provided the analysis. Purdom completed the data collection and provided statistical support. All authors contributed to the conceptualization and design and the manuscript preparation. Purdom can be reached at m_purdom@hotmail.com, with copy to editor at ONFEditor@ons.org. Submitted September 2016. Accepted for publication March 16, 2017.
References
Abdi, H., & Williams, L.J. (2010). Principal component analysis. Wiley Interdisciplinary Reviews: Computational Statistics, 2, 433–459.
American Nurses Association and International Association of Clinical Research Nurses. (2016). Clinical research nursing: Scope and standards of practice. Silver Spring, MD: American Nurses Association.
Bevans, M., Hastings, C., Wehrlen, L., Cusack, G., Matlock, A.M., Miller-Davis, C., . . . Wallen, G.R. (2011). Defining clinical research nursing practice: Results of a role delineation study. Clinical and Translational Science, 4, 421–427.
Carlson, C., Reilly, M., & Hitchens, A. (2005). An innovative approach to the care of patients on phase I and phase II clinical trials: The role of the experimental therapeutics nurse. Journal of Pediatric Oncology Nursing, 22, 353–364. doi:10.1177/1043454205281763
Castro, K., Bevans, M., Miller-Davis, C., Cusack, G., Loscalzo, F., Matlock, A.M., . . . Hastings, C. (2011). Validating the clinical research nursing domain of practice [Online exclusive]. Oncology Nursing Forum, 38, E72–E80. doi:10.1188/11.ONF.E72-E80
Chang, A.M., Gardner, G.E., Duffield, C., & Ramis, M.-A. (2012). Advanced practice nursing role development: Factor analysis of a modified role delineation tool. Journal of Advanced Nursing, 68, 1369–1379. doi:10.1111/j.1365-2648.2011.05850.x
Ehrenberger, H.E., & Lillington, L. (2004). Development of a measure to delineate the clinical trials nursing role [Online exclusive]. Oncology Nursing Forum, 31, E64–E68. doi:10.1188/04.ONF.E64-E68
Field, A. (2009). Discovering statistics using IBM SPSS Statistics. London, England: Sage.
Hastings, C.E., Fisher, C.A., McCabe, M.A., Allison, J., Brassil, D., Offenhartz, M., . . . Turbini, V. (2012). Clinical research nursing: A critical resource in the national research enterprise. Nursing Outlook, 60, 149–156. doi:10.1016/j.outlook.2011.10.003
International Association of Clinical Research Nurses. (2016). Specialty practice of clinical research nursing recognized by the American Nurses Association [Press release]. Retrieved from http://bit.ly/2vzXszO
Jones, C.T., & Wilson, L.L. (2013). Role perceptions of nurse clinical research coordinators. Nursing: Research and Reviews, 3(3), 133–139. doi:10.2147/NRR.S47579
Kaiser, H.F. (1974). An index of factorial simplicity. Psychometrika, 39, 31–36. doi:10.1007/BF02291575
Nagel, K., Gender, J., & Bonner, A. (2010). Delineating the role of a cohort of clinical research nurses in a pediatric cooperative clinical trials group [Online exclusive]. Oncology Nursing Forum, 37, E180–E185. doi:10.1188/10.ONF.E180-E185
Rieger, P.T., & Yarbro, C.H. (2003). Role of the oncology nurse. In D.W. Kufe, R.E. Pollock, R.R. Weichselbaum, R.C. Bast, T.S. Gansler, J.F. Holland, & E. Frei (Eds.), Cancer medicine (6th ed.). Hamilton, Ontario: BC Decker.
Scott, K., White, K., Johnson, C., & Roydhouse, J.K. (2011). Knowledge and skills of cancer clinical trials nurses in Australia. Journal of Advanced Nursing, 68, 1111–1121.
Spilsbury, K., Petherick, E., Cullum, N., Nelson, A., Nixon, J., & Mason, S. (2008). The role and potential contribution of clinical research nurses to clinical trials. Journal of Clinical Nursing, 17, 549–557. doi:10.111/j.1365-2702.2006.01872.x
U.S. Food and Drug Administration Responsibilities of Sponsors and Investigators Rule, 21 C.F.R. § 312, subpart D (2016). Retrieved from http://bit.ly/2rnf4t8

