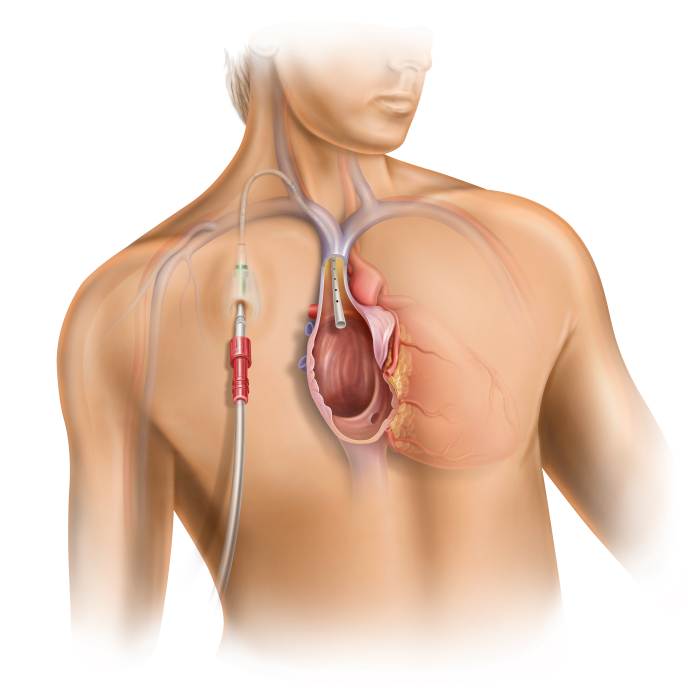
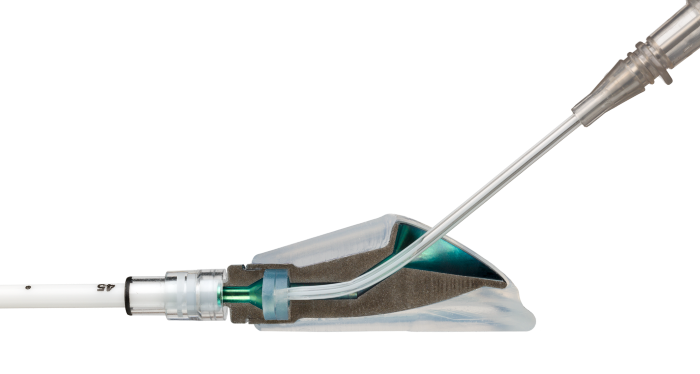
The High-Flow Port Designed & Indicated for Apheresis: PowerFlow™ Implantable Apheresis IV Port

With an ever-growing number of apheresis procedures and a rising chronic apheresis patient population, there is a need for long-term vascular access options. BD is a global leader in ports and we have listened to the challenges and needs of the apheresis community. Users asked for a durable device that can provide long-term access1 to the vascular system and deliver flow rates that apheresis procedures demand. The PowerFlow™ Implantable Apheresis IV Port is engineered to meet these needs, designed for long device life, maximum flow, and patient comfort.
The PowerFlow™ Port has a soft silicone body designed to help address patient comfort and contains a robust titanium funnel to assist with IV catheter access. The PowerFlow™ Port is indicated not only for apheresis therapies, but also for infusion therapies.
- Optimized for long device life – Bench tested up to 1,000 accesses1
- Engineered for maximum flow – Large 9.6F polyurethane catheter
- Bench tested for high-flow performance – Unique design and access delivers flow rates up to 150 mL/min at low pressure2
For patients, our PortReadyTM Program offers information about vascular access device options, including the PowerFlow™ Port. For healthcare professionals, the PortReadyTM Program gives you access to educational materials including videos, brochures, clinician training kits, and PortReadyTM displays. Why wait? Visit www.PortReady.com today.
As a healthcare professional, you have the opportunity to work with your patient to recommend the most appropriate vascular access device – You are your patient’s best advocate!
Learn more about the PowerFlowTM Port here: https://www.bd.com/en-us/products-and-solutions/products/product-families/powerflow-implantable-apheresis-iv-port
You can also watch this video about the PowerFlowTM Port: https://www.youtube.com/watch?v=EYJdB2hAClk
Visit the BD booth at the 2024 ONS Conference in Washington D.C. to learn more!
1 After 1000 IV catheter insertions, bench top leak testing was performed both with the device accessed (both 14G and 16G IV catheters tested separately) and with no IV catheter present. Bench testing may not be indicative of actual clinical performance. Different test methods may yield different results. Data on file, BD, Tempe, Arizona. 2 Mean flow rates, 25 cm catheter, when tested in a benchtop model using a blood simulant with viscosity of 3.5cP, N=27. Simulated testing may not be indicative of actual clinical performance. Changes in blood viscosity, catheter length, and IV type will affect achievable flow rates. Data on file, BD, Tempe, Arizona.
Please consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions and directions for use. BD, the BD Logo, PowerFlow are trademarks of Becton, Dickinson and Company or its affiliates. © 2024 BD. All rights reserved. BD-120563